GMP Solution
ICH-Q7 GMP Manufactured ProductSynthetic Glycerin, USP, EP,
Parenteral Grade, GMP Excipient Grade
Product Code: GLYS-3151
Intended For Use As A Parenteral Excipient
Synthetic Parenteral Glycerin (Glycerol) is a fully synthetic, parenteral grade, suitable for use in insulin manufacturing and other critical cGMP manufacturing applications and pharmaceutical preparations.
Product Specifications
| USP COMPENDIA | |||
|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
||
|
99.0 to 101.0% | ||
|
≤ 10ppm | ||
|
Passes Test | ||
|
Passes Test | ||
|
Conforms with reference standard ≤ 0.025%1 ≤ 0.025%1 Passes Test |
||
|
≤ 30ppm of Cl | ||
|
≤ 0.1% ≤ 0.5%1 |
||
|
≤ 0.01% |
||
| Specific gravity (at 25.0°C) |
≥ 1.249 |
||
| Sulfate |
≤ 20ppm |
||
| Water Content |
≤ 2.0%1 |
||
1Stringent specification
| EP COMPENDIA | |||
|---|---|---|---|
|
ANALYSIS |
SPECIFICATIONS |
||
|
≤ 10ppm | ||
|
Passes Test | ||
|
Clear, colorless, or almost colorless viscous liquid | ||
|
Solution is clear, colorless | ||
|
99.0 to 101.0%1 | ||
|
≤ 10ppm | ||
| Esters | Passes Test | ||
| Halogenated compounds | ≤ 35ppm | ||
|
1.470 – 1.475 Conforms with reference standard 1.258 - 1.268 |
||
|
≤ 0.025%2 |
||
| Sugars |
Passes Test |
||
| Sulfated Ash |
≤ 0.01% |
||
| Water |
≤ 2.0% |
||
1USP Specification
2Stringent specification
| List of C of As files |
|---|
| No files available right now. |

COAs and Tech Docs
- Printable Spec Sheet
- Product Inquiry
- C of As
- SDS
GLYS-3151
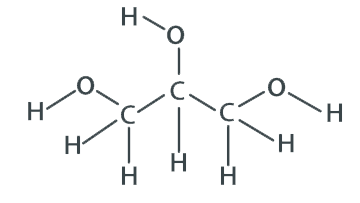
CAS #: 56-81-5
Formula: C3H8O3
F.W.: 92.1 g/mol
General Product Description:
Synthetic Glycerin is produced at our cGMP platform in India and then shipped to and fully retested and packaged at our sites in Rensselaer, NY and Bangor PA, USA.
-
Synthetic Glycerin is a Clear Liquid product.
-
Molecular Formula: C3 H8 O3
-
Molecular Weight: 92.09 g/mol
-
CAS Number: 56-81-5
-
There are no known major food allergens (as defined by FDA and WHO) in the manufacture of this product.
-
Synthetic Glycerin GLYS-3151 at BioSpectra and its raw materials are not derived from or come in contact with animal parts, products, and/or byproducts.
-
Synthetic Gycerin manufactured at our cGMP platform in India and any raw materials used in the manufacture of Synthetic Glycerin at BioSpectra are not subject to genetic modification.
-
Synthetic Gycerin is further purified at our site in Canada, Dextran Products apart of the BioSpectra Organization.
-
Synonyms: Glycerol, Glycerolum, Glicerol, Glyzerin, 1,2,3-Propantriol, Glycerinester, Pfl.
GMP Compliance:
Bio Excipient Grade Synthetic Glycerin, GLYS-3151 is suitable for use as an excipient. It is manufactured in accordance with the ICH-Q7 Good Manufacturing Practice Guide. This grade of Synthetic Glycerin is not suitable to be used as an Active Pharmaceutical Ingredient, Drug Product or Household Item.
Retest Date:
The recommended retest period for Synthetic Glycerin is two years from the date of manufacture.
Storage and Shipping Conditions:
Ship and store in ambient temperature.
Package Sizes:
6x1L, 4x 3.78L, 10L, 200L and 1000 liter
(Please contact us for more specific information about your package size and Chain of Custody.)
Country of Origin:
India
Additional Packaging Information
https://www.biospectra.us/technical/packaging


